History of the
SinusLogic™ Stent
Tubes and stents have a long history of use as medical devices. The SinusLogic Stent was developed using their proven method of function.
The Jones Tube
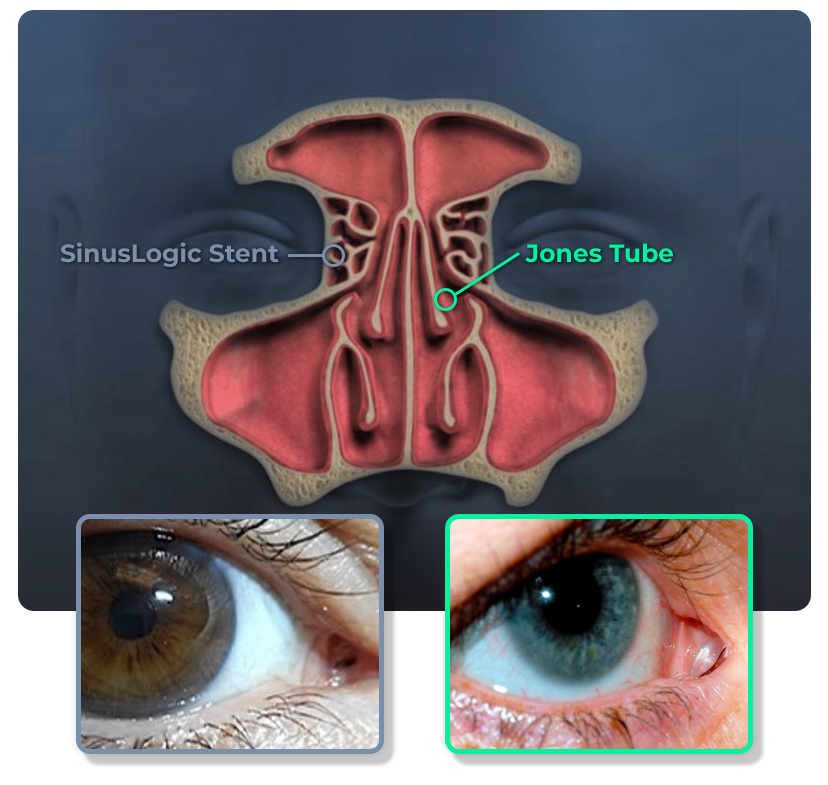
The SinusLogic Stent is predicated on a medical device called the Jones tube, a small, thin tube used for over 60 years to facilitate tear drainage when the normal tear pathway is blocked or damaged.
Our ENT founders discovered that using the same entrance as the Jones tube but placing the Stent at an angle posterior to the tear system creates a direct pathway into the anterior ethmoid sinus, allowing saline applied to the eye to drain “top-down” through the sinuses.
“Ear Tubes for the Sinuses”
The SinusLogic Stent is modeled similarly to ear tubes, but for the sinuses. Ear tubes are tiny hollow tubes that ENTs have used for over 70 years to treat chronic ear infections. They create a pathway for airflow through the eardrum into the middle ear which helps fluids drain into the throat or ear canal, making ear infections less frequent and troublesome.
Sinus Anatomy / Stent Placement
Regulatory Approvals
Since 2011, Sinopsys has worked with researchers, physicians, and regulatory professionals to realize SinusLogic Stent’s potential. Prior regulatory approvals include:
- FDA – 510(k) clearance to address ‘watery eye’ (lacrimal blockages)
- European Medical Agency CE Mark for treatment of Chronic Rhinosinusitis (CRS)
- FDA 510(k) De Novo Feasibility Clinical Study for CRS

