The Persistent Challenge of Chronic Sinusitis
Chronic Rhinosinusitis (CRS) is one of the most common long-term illnesses in the U.S. and affects nearly 30 million Americans annually.1 Symptoms include inflammation, nasal congestion, reduced sense of smell and facial pain and often results in decreased productivity and sleep, missed work days and diminished quality of life.
Unfortunately, 52% of patients fail to respond to standard treatments of oral antibiotics and steroids, steroid nasal sprays, and nasal saline rinses and many are reluctant to undergo invasive surgery with high revision rates.2

A New Pathway:
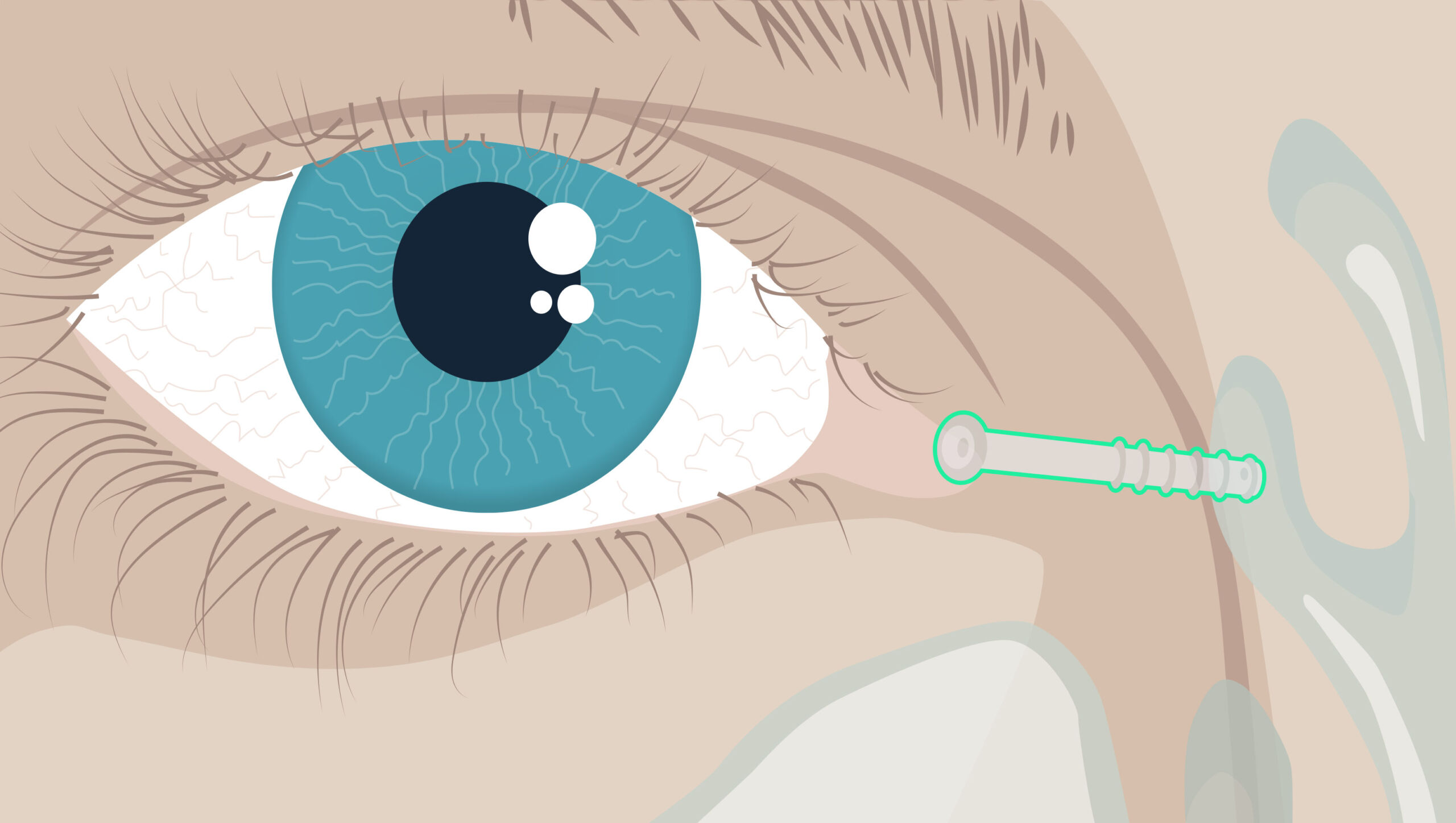
The SinusLogic™ Stent
Sinopsys has been approved for our US Pivotal Clinical Trial to evaluate the safety and efficacy of saline delivery through the SinusLogic™ Stent, for the treatment of Chronic Sinusitis patients.
The SinusLogic™ Stent is a small, flexible silicone tube placed in the soft tissue adjacent to the tear duct. The stent provides direct access to the ethmoid sinus – a common (often inaccessible) site of inflammation in CRS patients. By utilizing daily saline eye drops, this targeted means of irrigation may offer an effective treatment alternative, potentially reducing the frequent use of antibiotics and steroids.
Caution – Investigational Device, Limited by Federal (or United States) Law to Investigational Use.
The Significance of Saline in CRS Treatment
Saline has been a staple in medicine for centuries, used for cleaning wounds, reducing inflammation, rehydration and more.
Today, saline is a first-line treatment for chronic sinusitis patients3 as it dilutes mucus to promote clearing of antigens and bacterial biofilm and promotes inflammatory mediators to improve mucociliary function4. However, most “bottom-up” saline delivery approaches don’t successfully reach and rinse the critical frontal, sphenoid and ethmoid sinus cavities. As 89% of CRS cases involve the ethmoid sinus, it’s crucial that treatment effectively targets this area for optimal relief and long-term disease management5.


Dedicated to Finding New Solutions for Sinusitis Patients
Founded by physicians and advised by a highly skilled, experienced team of ENTs and healthcare executives, we are committed to developing novel therapies for our patients.
Our mission is to pioneer innovative treatments that provide lasting relief and improve the quality of life for those affected by sinusitis.
Dedicated to finding new solutions for chronic sinusitis
Founded by physicians and advised by a highly skilled, experienced team of ENTs and healthcare executives, we are committed to developing novel therapies for our patients.
Our mission is to pioneer innovative treatments that provide lasting relief and improve the quality of life for those affected by sinusitis.

What’s Next?
We have been approved by the FDA for our Pivotal Clinical Trial for the use of the SinusLogic Stent to irrigate sinus passageways with saline for treatment of Chronic Rhinosinusitis (CRS). Are you interested in learning more about our future clinical research study?
ENTs participating in focus groups stated the SinusLogic Stent would be offered to any patient failing medical therapy and before existing current endoscopic or balloon options.
– 2018 Sinopsys Focus Groups
FOOTNOTES:
- Diagnosis and treatment of chronic rhinosinusitis: focus on intranasal Amphotericin B
- Efficacy of medical therapy in treatment of chronic rhinosinusitis
- Clinical Practice Guideline: Nasal Irrigation for Chronic Rhinosinusitis in Adults
- Nasal Irrigation: An Imprecisely Defined Medical Procedure
- Characteristics Of Chronic Rhinosinusitis Phenotypes In Patients Undergoing Functional Endoscopic Sinus Surgery: An Observational Cohort Retrospective Study